Galvanizing is the immersion of a part made of steel or iron into a bath of molten metal, creating a metallurgical reaction between the part and the molten metal and producing a uniform thickness of the molten metal throughout the part. The process is resulting in producing a part that is corrosion resistant.
Several metals may be used in the molten bath to produce the required protective layer “Hot-dipping process“; however, zinc is the most commonly used metal for the hot-dipping process called hot-dip galvanizing.
Hot-dip galvanizing may use to protect all types of steel, alloy steels, cast steel, and cast iron.
Hot-dip Galvanizing Process
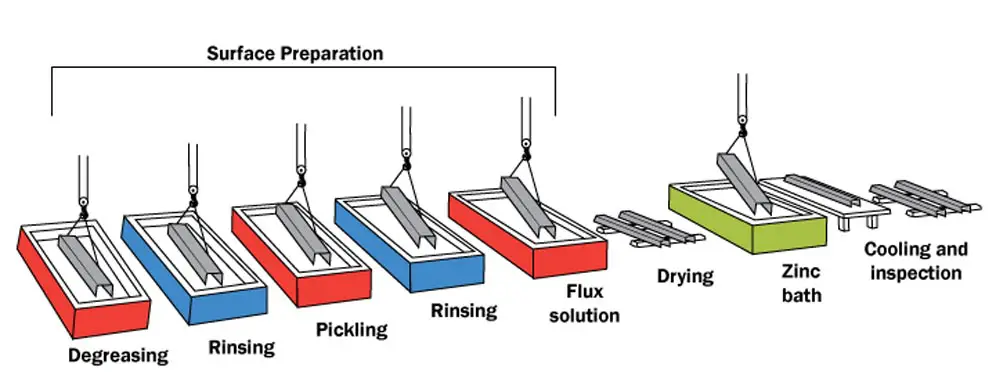
The hot-dip process consists of several stages that will go through it.
Surface Preparation
Surface preparation is the most crucial stage of the galvanizing process. The part should have a clean and clear surface free from mill-scale, welding slag, varnish, paint, oil, grease, and other contaminating residues.
Any residual oil and grease on the part may trap moisture. Moisture evaporates to steam during hot immersion causes miniature explosions in the zinc bath.
Zinc will not react with unclean steel; therefore, thorough surface preparation is essential. Two-step operation (Caustic Degreasing and Pickling) is the standard method to perform surface preparation.
Degreasing
The 1st stage is dipping the part into a warm degreasing bath. The process intends to remove paint, oil, grease, and other organic contaminating residues from the part’s surface before acid pickling.
Rinsing
This step comes after the caustic degreasing and pickling stages. Performed by washing the part with hot water to neutralize the part’s surface from its alkaline nature after caustic cleaning or acidic nature after pickling, making the part ready for its next stage.
Pickling
The 2nd stage is to immerse the part in a mineral acid bath (usually uses hydrochloric or sulfuric acid with a concentration of 10~15%). The process is intended to remove mill scale, rust, and other inorganic contaminating residues from the part’s surface.
Alternatively, dry abrasive blasting may be used after the degreasing stage, especially with cases as sand castings, to remove the burned-on sand generated from the casting process, which the pickling process will not able to remove.
Fluxing
The 3rd stage is fluxing; the process intends to remove surface oxides that may develop on the exposed part since the pickling process is completed and creates a temporary protective layer that prevents oxide formation before the dipping process starts. Also, fluxing enhances the wetting of the molten zinc onto the part’s substrate.
Fluxing is performed either dry or wet. Dry fluxing is to immerse the part in a heated solution of zinc ammonium chloride (the solution is called pre-flux solution with a concentration of 20~40%) before drying it in an oven (usually drying at 120ºC). Wet fluxing is conducted bypassing the part through a layer of molten zinc ammonium chloride, which floats on top of the molten zinc.
Galvanizing
The 4th stage is to dip the part into the molten zinc kettle. Depending on the part’s steel chemical composition, the part may remain in the zinc bath for 30 seconds up to eight hours at a temperature of 450~475°C till the galvanizing reaction is completed.
When the galvanizing reaction is completed, may either cool in the air or quenched in a water tank to eliminate further reaction between the steel and the zinc coating.
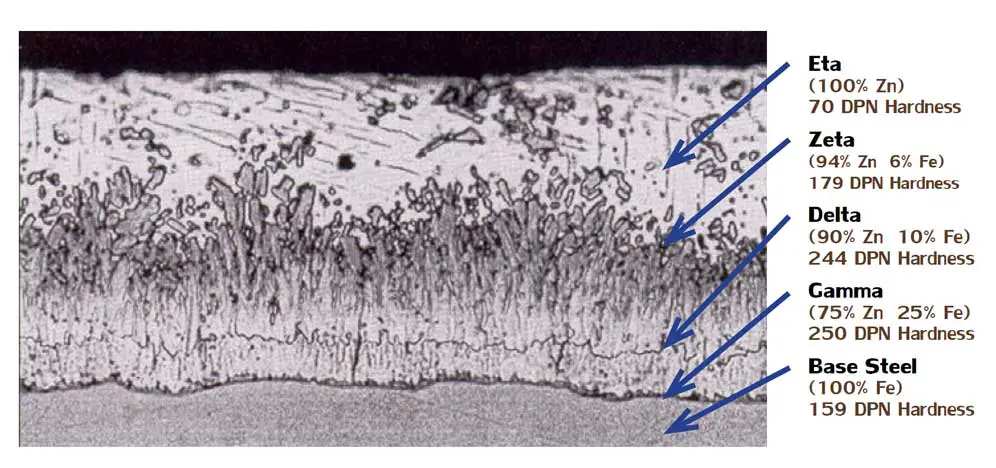
The galvanized coating consists of three distinct iron-zinc alloy compounds:
- Gamma (next to the steel) 75% zinc and 25% iron
- Delta 90% zinc and 10% iron
- Zeta 94% zinc and 6% iron
The outer layer, Eta, has the same composition as the bath—100% zinc.
Dipping in a zinc bath may be done in a “Single Dip” for small parts or “Double Dip” for large parts where the part is dipped from one end, then flipped and dipped from the other end.
Typically, a Zinc bath consists of at least 98% pure zinc with extra additives; additives are added to promote specific desired properties and enhances the galvanizing process.
Post Treatments
Several post-treatment methods are performed to enhance the final galvanized coating, such as:
- Expose the part while the zinc layer is still in the molten condition to air blasting, rolling, or centrifuging to reduce zinc coating thickness.
- Further appearance-enhancement by performing chromatin, phosphating, and light rolling to the part.
- Perform annealing to convert the zinc coating into alloy and change coating characteristics.
- Quenching in water with some chemical additives. Quenching produces a passive layer which protects the part from mechanical damages.
Inspection
The final stage is to perform a final inspection of the galvanized part and verify that the final result complies with the acceptance criteria.
Galvanizing Quality
The following quality remarks are related to galvanizing:
- Alternation of properties for workpiece: some steel grades may get affected by the galvanizing process especially quenching the steel after hot dipping.
- Workpiece design: workpiece design and fabrication may affect the quality of galvanizing; hence, workpiece size, welding location, pockets and holes in the workpiece, dissimilar metals, etc., are all points to consider when planning to galvanize some of the project parts.
- Workpiece Service life: Service life is measured by the thickness of the protective zinc, which is determined based mainly on atmospheric exposure.
- Adhesion: one of the crucial parameters to determine the galvanizing process’s quality, especially when galvanizing high-silicon steels that usually exhibit poor adhesion, “paring test” is a quick field measure test to measure galvanizing adhesion. The test is performed using a sharp knife and considerable pressure to attempt to remove a portion of the coating. Adhesion is usually considered satisfactory when it is possible to remove only small particles of the coating.
- Appearance: surface appearance requirement is to be continuous, smooth, and free from surface imperfections (e.g., cracks, bare spot, lumps, peeling, blisters, inclusions ash, or dross).
Galvanizing Inspection
Various inspection techniques are used in galvanizing inspection. The methods are explained in detail in the following standards: ASTM A123/A123M, A153/A153M, or A767/A767M.
The following are the most common:
- Coating Thickness – magnetic gauges, chemical stripping.
- Coating Weight – weigh-galvanize-weigh, and weigh-strip-weigh.
- Appearance – visual inspection.
- Additional Tests – (Stout knife, embrittlement, Chromatin, Bending).
Coating Thickness
Coating thickness may determine by chemical stripping or Magnetic thickness test.
Chemical stripping is measured by attaching a sample article to the workpiece during the hot-dipping process. The coated sample is adequately cleaned, dried, and weighed, and then immersed in a chemical solution to dissolves the zinc coating, dried, and weighed again. The weight is subtracted from the original weight, giving the zinc coating weight from which a predicted average coating thickness may be calculated.
The stripping methods give fairly accurate average coating weights of the zinc coating; however, it does not provide any information about how evenly the coating is distributed.
Magnetic thickness test uses magnetic thickness test instruments to determine specific spot thickness of the workpiece.
Galvanizing Appearance and Defects
Galvanized Surface appearance requirement is to be continuous, smooth, and free from surface imperfections. During the inspection, various surface conditions may observe that need to be evaluated and determine how it will affect the corrosion resistance performance.
Based on the inspection finding effect on the corrosion resistance performance, the surface remark/finding will consider accepted or rejected.
The following are the visual surface conditions the can be recorded during the inspection:
Bare Spots
The bare spot is an ungalvanized area of the workpiece; the defect is caused due to improper surface preparation (e.g., improper cleaning of paint, grease, oil, mill scale, rust scale, residual welding slag, lamination, laps, and folds, etc.) causing the zinc not to react with the base metal and producing missing areas.
General Roughness
Rough surfaces include coatings that have just a rough surface finish and can involve some groove-type surface configurations. A rough coating usually is caused by excessive growth or unevenness of the galvanizing layer. The galvanizing layer irregularity increases with the increase in thickness; therefore, heavy coatings are usually rough.
Dross Protrusion
Dross protrusions is a small, hard lump on a normal galvanized surface; it forms due to impurities on the zinc bath that float because of the dross layer’s agitation at the bottom of the bath. A clean zinc bath is less likely to produce this defect. The dross sticks to the coating layer, prevent drainage of the bath zinc in the immediate area, and a build-up occurs.
Lumpiness and Runs
A Lumpiness and runs are uneven coating results when withdrawing the galvanized article too fast or when the bath temperature is too low to allow surplus zinc to run back into the bath. Runs also may be caused by delayed drainage from various grooves and pockets of the part where zinc accumulates.
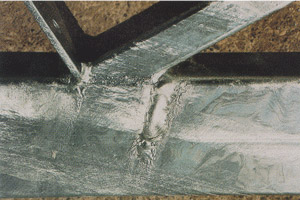
Flux Inclusions
Flux inclusions are zinc bath flux adherence to the galvanized part instead of separating cleanly from the surface as the work is dipped. Flux inclusions form black spots known by their tendency to pick up moisture.
Ash Inclusions
Ash inclusions are oxide film forms due to zinc ash; ash inclusive develops on the galvanizing bath’s surface. Ash may burn on the steel during dipping or picked up from the bath top during withdrawal. Ash inclusions can occur on heavy parts that require slow withdrawal from the bath.
Dull-gray Galvanized Coating
Dull-gray usually appears on heavy sections that cool slowly; on steel will high silicon, carbon, and phosphorus. The gray appearance develops during cooling and is caused by the diffusion of the zinc-iron alloy to the coating’s surface. It appears as isolated localized patches; however, it may extend over the entire surface in extreme cases.
Rust Stains
Rust stains may be caused by seepage from joints and seams after galvanizing or by the material being stored under or in contact with rusty steel.
After a short period of galvanized part exposure, a slight rusty appearance on the surface may appear on certain high-silicon content steels. It does not count as a galvanizing failure, but a phenomenon with this type of steel.
White Rust
Porous and bulky deposit that appears on the freshly galvanized part surface that closely stacked and damped in the poor ventilated condition during storage caused wet storage stain known as white rust.
Galvanizing Repair
Whenever one of the defects appears on the galvanized products, it must be repaired if allowed. Zinc-based solder, zinc-rich paint, and zinc spray metalizing are the material used to repair hot-dip galvanized products. ASTM A780 “Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coatings” provides guidelines on the repair methodologies to be followed during the repair.
Welding Galvanized Joint
Welding galvanized steel will require adequate ventilation and proper air circulation, especially in confined spaces. Welding shall be performed following American Welding Society Publication D19.0, 0-72 “Welding Zinc-Coated Steel” requirements.
References:
American Galvanizers Association.
NACE International, Coating Inspector Program Level II.